The highest occupied s and p sublevels are partially filled – The partially filled highest occupied s and p sublevels play a pivotal role in shaping the electronic configurations, chemical properties, and physical characteristics of elements. This comprehensive analysis delves into the intricacies of these sublevels, unraveling their significance and implications.
This discourse elucidates the concept of electronic configuration and demonstrates how Hund’s rule governs the electron distribution within partially filled sublevels. It further explores the energy level diagram and explains the ordering of s and p sublevels based on energy considerations.
Electronic Configuration

Electronic configuration refers to the distribution of electrons in different energy levels and orbitals around the atomic nucleus. Elements with the highest occupied s and p sublevels partially filled exhibit unique electronic configurations.
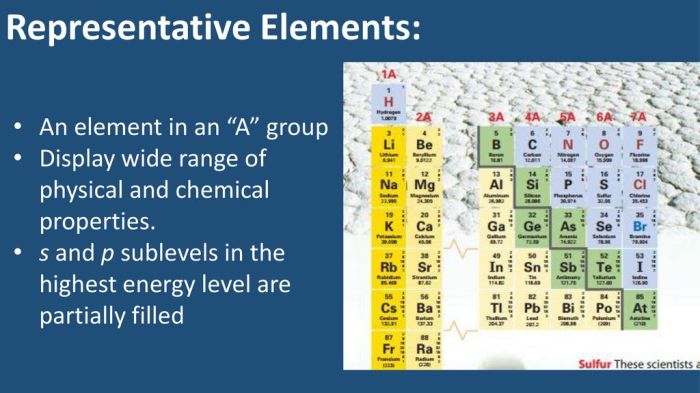
For instance, nitrogen (N) has the electronic configuration 1s 22s 22p 3. The highest occupied energy level is the second energy level (n=2), and the s and p sublevels are partially filled with two and three electrons, respectively.
Hund’s Rule
Hund’s rule states that the lowest energy configuration for an atom is the one with the maximum number of unpaired electrons in the same sublevel. For elements with partially filled s and p sublevels, Hund’s rule predicts that electrons will occupy individual orbitals within the sublevel before pairing up.
In the case of nitrogen, Hund’s rule dictates that the three electrons in the 2p sublevel will occupy three separate orbitals, each with one unpaired electron, resulting in the electronic configuration 1s 22s 22p 3.
Orbital Energy Levels
The energy level diagram for elements with partially filled s and p sublevels shows the relative energies of the orbitals. The s sublevel is generally lower in energy than the p sublevel, which means that electrons in the s sublevel are more tightly bound to the nucleus.
For example, in nitrogen, the 2s orbital is lower in energy than the 2p orbitals. Therefore, the two electrons in the 2s orbital are more strongly attracted to the nucleus than the three electrons in the 2p orbitals.
Valence Electrons
Valence electrons are the electrons in the outermost energy level of an atom. Elements with partially filled s and p sublevels have valence electrons in both the s and p sublevels.
In nitrogen, the valence electrons are the three electrons in the 2p sublevel. These valence electrons determine the chemical properties of nitrogen.
Chemical Properties
Elements with partially filled s and p sublevels tend to be chemically reactive. They can form covalent bonds by sharing valence electrons with other atoms.
For example, nitrogen can form a covalent bond with hydrogen to form ammonia (NH 3). In this compound, nitrogen shares its three valence electrons with three hydrogen atoms, resulting in a stable molecule.
Physical Properties, The highest occupied s and p sublevels are partially filled
Elements with partially filled s and p sublevels often have low melting and boiling points. This is because the valence electrons are not strongly attracted to the nucleus, which makes it easier for the atoms to move past each other.
For example, nitrogen is a gas at room temperature and pressure. This is due to the weak attraction between the valence electrons and the nucleus, which allows the nitrogen atoms to move freely.
Questions and Answers: The Highest Occupied S And P Sublevels Are Partially Filled
What is the significance of Hund’s rule in determining electron configuration?
Hund’s rule states that electrons occupy orbitals of equal energy with parallel spins before pairing occurs. This rule helps predict the ground state electronic configuration of atoms with partially filled sublevels.
How do the partially filled highest occupied s and p sublevels affect chemical reactivity?
The presence of unpaired electrons in these sublevels makes elements more reactive. These unpaired electrons can participate in chemical reactions, forming bonds with other atoms.
What is the relationship between electronic configuration and physical properties?
Electronic configuration influences physical properties such as electrical conductivity, magnetic susceptibility, and melting point. Elements with partially filled sublevels often exhibit paramagnetism and lower melting points due to the presence of unpaired electrons.